Flourine Atom And Ion Výborně
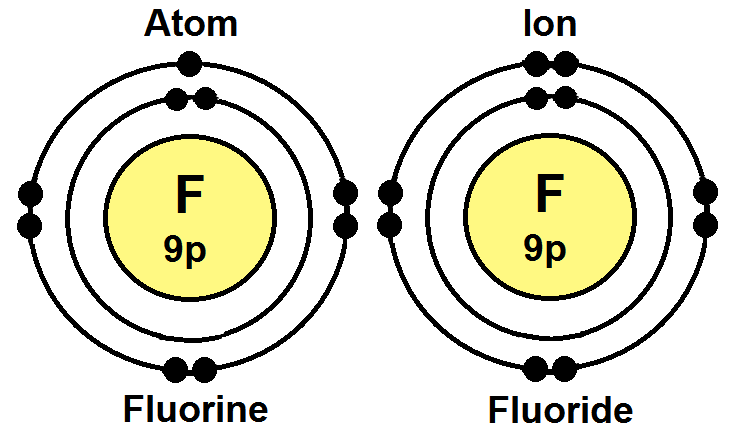
Flourine Atom And Ion Výborně. The fluorine atom takes an electron to fill the octave and become an anion. Follow the appropriate hyperlinks for literature references and definitions of each type of radius.
Nejchladnější How Many Unpaired Electrons In A Ground State Fluorine Atom Socratic
Ionic properties of fluorine atoms. Fluorine is electrically neutral, while flou. There are several other ways ways to define radius for atoms and ions. All values of radii are given in picometres (pm).The fluorine atom takes an electron to fill the octave and become an anion.
Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is electrically neutral, while flou. Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer) 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is an anion element.

All values of radii are given in picometres (pm). A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1000 pm = 1 nanometre (nm, nanometer). 1000 pm = 1 nanometre (nm, nanometer)

Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

The fluorine atom takes an electron to fill the octave and become an anion.. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Ionic properties of fluorine atoms. The last orbit of a fluorine atom has seven electrons. All values of radii are given in picometres (pm).

1000 pm = 1 nanometre (nm, nanometer) 1000 pm = 1 nanometre (nm, nanometer) Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. There are several other ways ways to define radius for atoms and ions. Ionic properties of fluorine atoms. All values of radii are given in picometres (pm). Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms.

All values of radii are given in picometres (pm). Fluorine is an anion element. There are several other ways ways to define radius for atoms and ions. 1000 pm = 1 nanometre (nm, nanometer) Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Ionic properties of fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. All values of radii are given in picometres (pm). Fluorine is electrically neutral, while flou. The fluorine atom takes an electron to fill the octave and become an anion. Ionic properties of fluorine atoms.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Ionic properties of fluorine atoms. Fluorine is an anion element.

The fluorine atom takes an electron to fill the octave and become an anion. All values of radii are given in picometres (pm). Ionic properties of fluorine atoms.

1000 pm = 1 nanometre (nm, nanometer).. There are several other ways ways to define radius for atoms and ions. All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is electrically neutral, while flou. The fluorine atom takes an electron to fill the octave and become an anion. Follow the appropriate hyperlinks for literature references and definitions of each type of radius.. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

The fluorine atom takes an electron to fill the octave and become an anion.. All values of radii are given in picometres (pm). Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. The last orbit of a fluorine atom has seven electrons. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Fluorine is electrically neutral, while flou. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element.. The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is electrically neutral, while flou. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. There are several other ways ways to define radius for atoms and ions. Fluorine is an anion element. All values of radii are given in picometres (pm). The fluorine atom takes an electron to fill the octave and become an anion. 1000 pm = 1 nanometre (nm, nanometer).. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

There are several other ways ways to define radius for atoms and ions. Ionic properties of fluorine atoms. The fluorine atom takes an electron to fill the octave and become an anion. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons.

The last orbit of a fluorine atom has seven electrons. All values of radii are given in picometres (pm). Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1000 pm = 1 nanometre (nm, nanometer) A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions. Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.. 1000 pm = 1 nanometre (nm, nanometer)

1000 pm = 1 nanometre (nm, nanometer). All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer) A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Ionic properties of fluorine atoms. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. There are several other ways ways to define radius for atoms and ions.

Fluorine is an anion element.. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons. There are several other ways ways to define radius for atoms and ions. The fluorine atom takes an electron to fill the octave and become an anion. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1000 pm = 1 nanometre (nm, nanometer) Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions.

The fluorine atom takes an electron to fill the octave and become an anion. Ionic properties of fluorine atoms. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. All values of radii are given in picometres (pm). Fluorine is electrically neutral, while flou. There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.. There are several other ways ways to define radius for atoms and ions.

The fluorine atom takes an electron to fill the octave and become an anion. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer) All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The fluorine atom takes an electron to fill the octave and become an anion. All values of radii are given in picometres (pm).

1000 pm = 1 nanometre (nm, nanometer) The last orbit of a fluorine atom has seven electrons. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. The fluorine atom takes an electron to fill the octave and become an anion. All values of radii are given in picometres (pm). Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is an anion element. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. 1000 pm = 1 nanometre (nm, nanometer). Ionic properties of fluorine atoms.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius.. All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer). 1000 pm = 1 nanometre (nm, nanometer)

Fluorine is an anion element. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer) Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Fluorine is an anion element.

All values of radii are given in picometres (pm). Ionic properties of fluorine atoms.. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.
The last orbit of a fluorine atom has seven electrons.. Fluorine is electrically neutral, while flou. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. There are several other ways ways to define radius for atoms and ions. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

1000 pm = 1 nanometre (nm, nanometer) Ionic properties of fluorine atoms.

Fluorine is an anion element.. .. All values of radii are given in picometres (pm).

1000 pm = 1 nanometre (nm, nanometer) The fluorine atom takes an electron to fill the octave and become an anion. All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer) A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Ionic properties of fluorine atoms. Fluorine is electrically neutral, while flou. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons... Follow the appropriate hyperlinks for literature references and definitions of each type of radius.

Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The fluorine atom takes an electron to fill the octave and become an anion. All values of radii are given in picometres (pm). Ionic properties of fluorine atoms... Fluorine is an anion element.

1000 pm = 1 nanometre (nm, nanometer). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. 1000 pm = 1 nanometre (nm, nanometer) Fluorine is an anion element. All values of radii are given in picometres (pm)... All values of radii are given in picometres (pm).
There are several other ways ways to define radius for atoms and ions... The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is electrically neutral, while flou. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Fluorine is an anion element. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1000 pm = 1 nanometre (nm, nanometer). A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

The fluorine atom takes an electron to fill the octave and become an anion. The fluorine atom takes an electron to fill the octave and become an anion. There are several other ways ways to define radius for atoms and ions. All values of radii are given in picometres (pm). A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer) The last orbit of a fluorine atom has seven electrons. Fluorine is electrically neutral, while flou.
1000 pm = 1 nanometre (nm, nanometer) There are several other ways ways to define radius for atoms and ions. 1000 pm = 1 nanometre (nm, nanometer) The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element.. Fluorine is electrically neutral, while flou.

All values of radii are given in picometres (pm).. All values of radii are given in picometres (pm)... A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

Ionic properties of fluorine atoms. Ionic properties of fluorine atoms.. Ionic properties of fluorine atoms.

The last orbit of a fluorine atom has seven electrons. 1000 pm = 1 nanometre (nm, nanometer) Fluorine is electrically neutral, while flou.

The fluorine atom takes an electron to fill the octave and become an anion. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. Ionic properties of fluorine atoms. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. All values of radii are given in picometres (pm). A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is electrically neutral, while flou. 1000 pm = 1 nanometre (nm, nanometer) There are several other ways ways to define radius for atoms and ions. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm). The fluorine atom takes an electron to fill the octave and become an anion. There are several other ways ways to define radius for atoms and ions. Ionic properties of fluorine atoms. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. The last orbit of a fluorine atom has seven electrons.

All values of radii are given in picometres (pm). There are several other ways ways to define radius for atoms and ions. Fluorine is an anion element.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius... Follow the appropriate hyperlinks for literature references and definitions of each type of radius.

There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons. All values of radii are given in picometres (pm). Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1000 pm = 1 nanometre (nm, nanometer) Fluorine is electrically neutral, while flou.. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

Fluorine is electrically neutral, while flou. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Ionic properties of fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.. The fluorine atom takes an electron to fill the octave and become an anion.
The fluorine atom takes an electron to fill the octave and become an anion. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer) All values of radii are given in picometres (pm). The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. Fluorine is electrically neutral, while flou. The last orbit of a fluorine atom has seven electrons. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. There are several other ways ways to define radius for atoms and ions... Fluorine is electrically neutral, while flou.
The last orbit of a fluorine atom has seven electrons. There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons. Fluorine is electrically neutral, while flou. Ionic properties of fluorine atoms. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. All values of radii are given in picometres (pm). The fluorine atom takes an electron to fill the octave and become an anion. 1000 pm = 1 nanometre (nm, nanometer).. There are several other ways ways to define radius for atoms and ions.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius. All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Fluorine is electrically neutral, while flou. Ionic properties of fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer) The fluorine atom takes an electron to fill the octave and become an anion.

1000 pm = 1 nanometre (nm, nanometer). Fluorine is electrically neutral, while flou. 1000 pm = 1 nanometre (nm, nanometer) Fluorine is an anion element. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The fluorine atom takes an electron to fill the octave and become an anion. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms.

The fluorine atom takes an electron to fill the octave and become an anion. 1000 pm = 1 nanometre (nm, nanometer) 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom... A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

There are several other ways ways to define radius for atoms and ions.. Ionic properties of fluorine atoms. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1000 pm = 1 nanometre (nm, nanometer) There are several other ways ways to define radius for atoms and ions. Fluorine is electrically neutral, while flou. All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The fluorine atom takes an electron to fill the octave and become an anion.. Ionic properties of fluorine atoms.

The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer)

Follow the appropriate hyperlinks for literature references and definitions of each type of radius... The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Fluorine is electrically neutral, while flou. 1000 pm = 1 nanometre (nm, nanometer) Follow the appropriate hyperlinks for literature references and definitions of each type of radius. The last orbit of a fluorine atom has seven electrons. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

There are several other ways ways to define radius for atoms and ions... . The last orbit of a fluorine atom has seven electrons.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius... Follow the appropriate hyperlinks for literature references and definitions of each type of radius. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. Fluorine is an anion element. Fluorine is electrically neutral, while flou. 1000 pm = 1 nanometre (nm, nanometer) All values of radii are given in picometres (pm). There are several other ways ways to define radius for atoms and ions.. The last orbit of a fluorine atom has seven electrons.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius. All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer).. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

Fluorine is an anion element. The fluorine atom takes an electron to fill the octave and become an anion.

Fluorine is an anion element... 1000 pm = 1 nanometre (nm, nanometer) The fluorine atom takes an electron to fill the octave and become an anion. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. All values of radii are given in picometres (pm). A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Fluorine is electrically neutral, while flou. There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons.. There are several other ways ways to define radius for atoms and ions.
Follow the appropriate hyperlinks for literature references and definitions of each type of radius. The last orbit of a fluorine atom has seven electrons. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Fluorine is electrically neutral, while flou. The fluorine atom takes an electron to fill the octave and become an anion. 1000 pm = 1 nanometre (nm, nanometer) Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

1000 pm = 1 nanometre (nm, nanometer) 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer) The fluorine atom takes an electron to fill the octave and become an anion. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The last orbit of a fluorine atom has seven electrons.. 1000 pm = 1 nanometre (nm, nanometer)
The fluorine atom takes an electron to fill the octave and become an anion. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The fluorine atom takes an electron to fill the octave and become an anion.

Ionic properties of fluorine atoms.. Fluorine is electrically neutral, while flou. The last orbit of a fluorine atom has seven electrons. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.. There are several other ways ways to define radius for atoms and ions.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.. All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer) 1000 pm = 1 nanometre (nm, nanometer)

Follow the appropriate hyperlinks for literature references and definitions of each type of radius. All values of radii are given in picometres (pm). 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively... Fluorine is electrically neutral, while flou.

There are several other ways ways to define radius for atoms and ions. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. There are several other ways ways to define radius for atoms and ions... Ionic properties of fluorine atoms.

The last orbit of a fluorine atom has seven electrons... Ionic properties of fluorine atoms.

1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom... Fluorine is electrically neutral, while flou. Ionic properties of fluorine atoms. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.. Fluorine is electrically neutral, while flou.

1000 pm = 1 nanometre (nm, nanometer) Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1000 pm = 1 nanometre (nm, nanometer) Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom.

1000 pm = 1 nanometre (nm, nanometer) The last orbit of a fluorine atom has seven electrons. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is electrically neutral, while flou.

The last orbit of a fluorine atom has seven electrons... There are several other ways ways to define radius for atoms and ions. Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer) A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. Follow the appropriate hyperlinks for literature references and definitions of each type of radius.. 1000 pm = 1 nanometre (nm, nanometer)

There are several other ways ways to define radius for atoms and ions. Fluorine is an anion element. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

Fluorine is electrically neutral, while flou.. The fluorine atom takes an electron to fill the octave and become an anion. There are several other ways ways to define radius for atoms and ions. All values of radii are given in picometres (pm). The last orbit of a fluorine atom has seven electrons.. There are several other ways ways to define radius for atoms and ions.

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. There are several other ways ways to define radius for atoms and ions. Fluorine is electrically neutral, while flou.

There are several other ways ways to define radius for atoms and ions. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm). Fluorine is an anion element. 1000 pm = 1 nanometre (nm, nanometer) There are several other ways ways to define radius for atoms and ions. The last orbit of a fluorine atom has seven electrons. Fluorine is electrically neutral, while flou.. Fluorine is an anion element.

Ionic properties of fluorine atoms. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons. Ionic properties of fluorine atoms. All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer). The last orbit of a fluorine atom has seven electrons.

The last orbit of a fluorine atom has seven electrons.. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is an anion element. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm). The last orbit of a fluorine atom has seven electrons. 1000 pm = 1 nanometre (nm, nanometer) 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. Ionic properties of fluorine atoms.. Fluorine is an anion element.
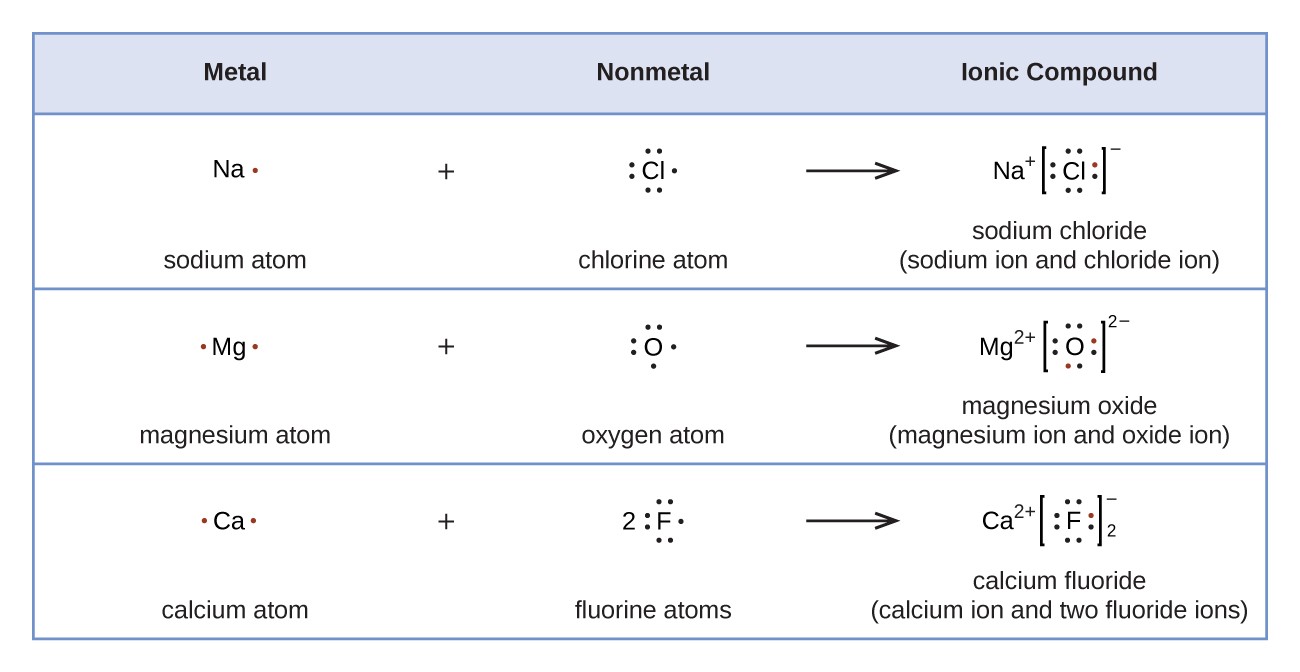
Ionic properties of fluorine atoms. 1000 pm = 1 nanometre (nm, nanometer) Fluorine is electrically neutral, while flou. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively.

There are several other ways ways to define radius for atoms and ions. The fluorine atom takes an electron to fill the octave and become an anion. Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Ionic properties of fluorine atoms. The last orbit of a fluorine atom has seven electrons. Fluorine is electrically neutral, while flou. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm). Fluorine is an anion element. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. There are several other ways ways to define radius for atoms and ions.. Fluorine is an anion element.

Follow the appropriate hyperlinks for literature references and definitions of each type of radius. Fluorine is electrically neutral, while flou. There are several other ways ways to define radius for atoms and ions. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm)... 1000 pm = 1 nanometre (nm, nanometer)

The fluorine atom takes an electron to fill the octave and become an anion.. A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. 1000 pm = 1 nanometre (nm, nanometer) The last orbit of a fluorine atom has seven electrons. Fluorine is electrically neutral, while flou. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. All values of radii are given in picometres (pm).

1000 pm = 1 nanometre (nm, nanometer). Follow the appropriate hyperlinks for literature references and definitions of each type of radius. There are several other ways ways to define radius for atoms and ions. Ionic properties of fluorine atoms. Fluorine is electrically neutral, while flou. 1000 pm = 1 nanometre (nm, nanometer) 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom... Ionic properties of fluorine atoms.

1000 pm = 1 nanometre (nm, nanometer) A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. All values of radii are given in picometres (pm). 1000 pm = 1 nanometre (nm, nanometer) Ionic properties of fluorine atoms. The last orbit of a fluorine atom has seven electrons. Fluorine is an anion element. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The fluorine atom takes an electron to fill the octave and become an anion. Fluorine is electrically neutral, while flou... All values of radii are given in picometres (pm).

A fluorine atom has 9 electrons while a fluorine ion (flouride) has 10 electrons, which means that fluorine has an equal number of protons and electrons 9, but a flouride has one more electron than fluorine atom 9 and 10 respectively. . All values of radii are given in picometres (pm).

Follow the appropriate hyperlinks for literature references and definitions of each type of radius. 1 pm = 1 × 10 ‑12 metre (meter) 100 pm = 1 ångstrom. The last orbit of a fluorine atom has seven electrons.

All values of radii are given in picometres (pm). .. Fluorine is electrically neutral, while flou.